ABSTRACT
Objective: To investigate the effect of the small molecule, RepSox (E-616452), on the expression of developmentally important genes and the pre-implantation development of rhesus monkey-pig interspecies somatic cell nuclear transfer (iSCNT) embryos.
Results: Rhesus monkey cells expressing the monomeric red fluorescent protein 1 which have a normal (42) chromosome complement, were used as donor cells to generate iSCNT embryos. RepSox increased the expression levels of the pluripotency-related genes, Oct4 and Nanog (p < 0.05), but not of Sox2 compared with untreated embryos at the 2-4-cell stage. Expression of the anti-apoptotic gene, Bcl2, and the pro-apoptotic gene Bax was also affected at the 2-4-cell stage. RepSox treatment also increased the immunostaining intensity of Oct4 at the blastocyst stage (p < 0.05). Although the blastocyst developmental rate was higher in the group treated with 25 μM RepSox for 24 hours than in the untreated control group (2.4 vs. 1.2%, p > 0.05), this was not significant.
Conclusion: RepSox can improve the developmental potential of rhesus monkey-pig iSCNT embryos by regulating the expression of pluripotency-related genes.
Keywords: Embryos (monkey-pig), Interspecies (monkey-pig) somatic cell nuclear transfer, mRFP1, Pluripotency, RepSox, Rhesus monkey
INTRODUCTION
Several molecules that can efficiently reprogram cells to generate induced pluripotent stem cell (iPSC) lines have been used to enhance nuclear reprogramming by regulating the expression of specific genes and to improve the developmental competence of somatic cell nuclear transfer (SCNT) embryos, such as trichostatin A, valproic acid, 5-aza-cytidine and BIX-01294 (Hou et al. 2013; Yamanaka et al. 2009; Kang et al. 2013; Tsuji et al. 2009; Huang et al. 2009). Although small molecules can improve the efficiency of SCNT embryo development in vitro and in vivo, conflicting results have been reported when interspecies SCNT (iSCNT) embryos were treated with small molecules (Shi et al. 2008; Srirattana et al. 2008). Trichostatin A does not improve the development of rabbit-human interspecies cloned embryos (Shi et al. 2008). Treatment with valproic acid and trichostatin A does not significantly improve the development of Przewalski’s gazelle-bovine iSCNT embryos (Zuo et al. 2014). A novel small molecule, RepSox (E-616452), a transforming growth factor-β receptor 1 kinase inhibitor, improves the efficiency of pluripotency gene transduction for reprogramming somatic cells into iPSCs (Ichida et al. 2009). Treatment with RepSox allows the generation of iPSCs from both adult and embryonic fibroblasts with a frequency comparable to that achieved by Sox2 transduction (Hou et al. 2013). To date, the effect of RepSox treatment on iSCNT embryos has not been reported.
In this study, we explored for the first time the effect of RepSox on the in vitro nuclear reprogramming competence of rhesus monkey-pig iSCNT embryos. We employed monomeric red fluorescent protein 1 (mRFP1)-expressing rhesus monkey fibroblasts as donor cells for iSCNT. The relative mRNA levels of important pluripotency-related (Oct4, Nanog, and Sox2) and apoptosis-related (Bax and Bcl2) genes were examined in iSCNT embryos. We also assessed the immunostaining intensity of Oct4 in cloned iSCNT embryos at the blastocyst stage. In addition, the influence of the culture medium on rhesus monkey-pig iSCNT embryo development was determined. This study provides a basis for further achievement regarding the generation of primate organs in pigs via interspecies blastocyst complementation.
MATERIALS AND METHODS
Animals
This study was carried out in strict accordance with the guidelines for the care and use of animals of Yanbian University. All animal experimental procedures were approved by the Committee on the Ethics of Animal Experiments at Yanbian University (Approval ID 20160128).
Chemicals
All chemicals and reagents used in this study were purchased from Sigma unless otherwise noted. RepSox was synthesized by WUXI APPTEC (Tianjin, China) (Hou et al. 2013).
Karyotyping of mRFP1-expressing Rhesus Monkey Cells
Rhesus monkey fibroblasts expressing mRFP1 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 20% (v/v) fetal bovine serum (FBS) until they reached 70-80% confluency, after which they were treated with 0.5 μg colcemid/ml for 4 hours in 5% CO2 at 38°C to arrest cell division at metaphase. Arrested cells were treated with a hypotonic solution of 75 μM KCl for 20 minutes at 37°C. Swollen cells were fixed in methanol/acetic acid (3:1 v/v) for 30 minutes at 4°C, and then centrifuged for 10 minutes at approximately 2000×g. The fixation procedure was repeated three times. In the last repeat, cells were kept in the fixative and placed onto pre-chilled glass slides. Chromosome spreads were kept at 25°C for 2 days, held at 65°C for 4 hours, stained with 1% Giemsa solution for 15 minutes, and imaged.
Preparation of Donor Cells
mRFP1-expressing rhesus monkey fibroblasts used for iSCNT were prepared as previously described (Zhu et al. 2014). Briefly, abdominal tissue was obtained from an adult rhesus monkey during trauma treatment at Yanji Zoo (Yanji, China). The tissue was cut into small pieces and cultured in DMEM containing 20% (v/v) FBS, 100 μg penicillin/ml, and 100 μg streptomycin/ml, in a humidified atmosphere of 5% CO2 at 38.5°C. Cells were observed around the pieces of tissue after 3-5 days. The medium was then replaced every 2 days until a fibroblast layer was established (7-10 days). The cells were then passaged three to four times. Electroporation was performed using the Lonza Nucleofector system (Lonza Biologics, Cologne, Germany). Ear fibroblasts (approximately 1.4 × 106 cells) were electroporated with 3 μg linearized DNA pCX-mRFP1-pgk-neoR vector (kindly provided by Dr. Xiaohui Wu, Fudan University, Shanghai, China) (Zhu et al. 2005) using the Amaxa Basic Nucleofector for primary fibroblasts kit (VPI-1002, Lonza), program V-026. After electroporation, cells were resuspended in 2 ml cell culture medium and cultured in 5% CO2 at 38°C. After 48 hours, 200 μg G418/ml was added to the medium and cells were cultured for a further 12 days to select transfected cells. Dishes were observed under ultraviolet light. Colonies with a high level of uniform fluorescence were picked and transferred to 96-well plates. Cells were cultured in DMEM containing 10% (v/v) FBS in a humidified atmosphere of 5% CO2 at 38.5°C. The cells were then passaged three to four times until approximately 80% confluent after which iSCNT was performed.
In Vitro Maturation of Porcine Oocytes
Ovaries were collected from prepubertal gilts at a local slaughterhouse and transported to the laboratory at 25-35°C. Antral follicles (2-6 mm in diameter) were aspirated using an 18-gauge needle. Aspirated oocytes that had a uniformly granulated cytoplasm and were surrounded by at least three uniform layers of compact cumulus cells were selected and washed three times in HEPES-buffered NCSU-37 medium containing 0.1% polyvinyl alcohol (PVA). Oocytes were cultured in four-well plates for 20 hours, with each well containing 500 μl NCSU-37 medium supplemented with 10% (v/v) porcine follicular fluid, 0.6 mM cysteine, 1 mM dibutyryl cyclic adenosine monophosphate (dbcAMP), and 0.1 IU human menopausal gonadotropin/ml (hMG; Teikokuzoki, Tokyo, Japan), followed by culture in the absence of dbcAMP and hMG for a further 18-24 hours.
Nuclear Transfer
Nuclear transfer was performed as described previously (Yin et al. 2002). Briefly, mature eggs that had formed the first polar body were cultured in medium supplemented with 0.4 mg demecolcine/ml and 0.05 M sucrose for 1 hour. (Sucrose was used to enlarge the perivitelline space.) Oocytes with a protruding membrane were moved to medium supplemented with 5 mg cytochalasin B (CB)/ml and 0.4 mg demecolcine/ml, and the protrusion was removed using a beveled pipette. A single donor cell was injected into the perivitelline space of each oocyte, which was then electrically fused using two direct current pulses of 150 V/mm for 50 μs in 0.28 M mannitol supplemented with 0.1 mM MgSO4 and 0.01% PVA. Fused oocytes were cultured in NCSU-37 medium for 1 hour, electro-activated, and then cultured in 5 mg CB-supplemented medium/ml for 4 hours. Reconstructed oocytes were activated by two direct current pulses of 100 V/mm for 20 μs in 0.28 M mannitol supplemented with 0.1 mM MgSO4 and 0.05 mM CaCl2. Activated eggs were cultured in this medium for 6 days in 5% (v/v) CO2 and 95% air at 39°C. Finally, blastocysts were placed onto a drop of glycerol/phosphate-buffered saline (PBS, 9:1) containing 20 μg Hoechst 33342/ml on a microscope slide. A coverslip was placed on top of the blastocysts, and the edge was sealed with nail polish. The nuclei were counted under ultraviolet light. Activated iSCNT embryos were cultured in medium for 7 days.
Culture of iSCNT Embryos
Activated rhesus monkey-pig iSCNT embryos were placed in either NCSU-37 medium (Coy et al. 2002), which is usually used for pig embryos, HECM-9 medium (Tachibana et al. 2012), which is used for monkey embryos, or iPSC medium, which is used for iPSC culture (Okita et al. 2007).
Immunofluorescence Staining for Oct4
RepSox-treated and non-treated iSCNT embryos were washed three times in 1% PVA-supplemented PBS, fixed with PBS containing 4% (v/v) paraformaldehyde for 45 minutes, and permeabilized with PBS containing 1% Triton X-100 at 37°C for 30 minutes. The permeabilized embryos were incubated for 1 hour in PBS supplemented with 2% (w/v) bovine serum albumin for blocking. Thereafter, the embryos were incubated with a rabbit polyclonal primary antibody against Oct4 (1:200; Santa Cruz Biotechnology) overnight at 4°C. A goat anti-rabbit fluorescein isothiocyanate-conjugated secondary antibody (1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) was then applied for 1 hour at room temperature. After washing three times in PBS, DNA was counterstained with 10 μg Hoechst 33342/ml for 10 minutes. Stained embryos were then mounted under a coverslip with antifade mounting medium to retard photobleaching. The experiments were replicated at least three times, and five and six embryos were processed in RepSox-treated and non-treated groups, respectively. Slides were scanned using an epifluorescence microscope (Nikon) to monitor fluorescein isothiocyanate at 488 nm. Images were captured and quantified using Nikon NIS element software.
Quantitative Real-time PCR
Total RNA was extracted from groups of 150 rhesus monkey-pig iSCNT 2-4-cell-stage embryos treated with or without RepSox using the Dynabeads mRNA direct kit (Dynal Asa, Oslo, Norway) according to the manufacturer’s instructions. First-strand complementary DNA (cDNA) was synthesized by reverse transcription of mRNA using the oligo(dT) 12-18 primer and SuperScript III reverse transcriptase (Invitrogen). Real-time PCR amplification was conducted with a QuantiTect SYBR Green PCR kit (Finnzymes, Espoo, Finland) on a RotorGene 2000 real-time PCR system (Applied Biosystems). Each real-time PCR mixture contained 1 μl cDNA, 10 μl SYBR, 0.5 μl ROX, 7.5 μl nuclease-free water and 0.5 μl appropriate forward and reverse primers (see Supplementary Table 1) in 20 μl. All tests were conducted in triplicate. Relative gene expression data were analyzed using quantitative real-time PCR and the 2−ΔΔCT method.
Experimental Designs
In Experiment 1, the effect of the concentration and exposure time of RepSox on the development of mRFP1-expressing rhesus monkey-pig iSCNT cloned embryos was determined. After activation for 4 hours, iSCNT embryos were treated with various concentrations of RepSox (0, 5, 25, or 50 μM) for 24 hours. iSCNT embryos were cultured in medium supplemented with 25 μM RepSox for 0, 1, 2, 4, and 7 days and transferred to medium without RepSox. Cleavage and blastocyst formation were evaluated on Days 2 and 7, respectively, with the day of iSCNT designated as Day 1.
In Experiment 2, the influence of culture medium on rhesus monkey-pig iSCNT embryo development was determined. Activated iSCNT embryos were placed in NCSU-37, HECM-9, or iPSC medium. Cleavage and blastocyst formation were evaluated on Days 2 and 7, respectively, with the day of iSCNT designated as Day 1.
In Experiment 3, immunodetection of Oct4 in rhesus monkey-pig iSCNT embryos at the blastocyst stage was performed. iSCNT embryos were treated with or without RepSox and then collected at the blastocyst stage. The average fluorescence intensities of Oct4 labeling were compared between the RepSox-treated and untreated groups.
In Experiment 4, the mRNA expression levels of apoptosis- and totipotency-related genes at the 2-4-cell stage were determined. iSCNT embryos were treated with or without RepSox and collected at the 2-4-cell stage. Relative mRNA expression of pluripotency-related (Oct4, Sox2, and Nanog) and apoptosis-related (Bax and Bcl2) genes was compared between the RepSox-treated and untreated groups.
Statistical Analysis
All data were obtained from more than three replicates. Data expressed as proportions (i.e., percentages) were analyzed using the chi-square test, and nuclei numbers were analyzed by the T-test (independent samples) using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). The average fluorescence intensity of each individual nucleus was quantified using Nikon NIS element software. P values < 0.05 were regarded as statistically significant.
RESULTS
Effect of RepSox Treatment on the In Vitro Development of mRFP1-expressing Rhesus Monkey-Pig iSCNT Embryos
mRFP1-expressing rhesus monkey fibroblast cells were used as donor cells for rhesus monkey-pig iSCNT (Figure 1a, b). Karyotype analysis demonstrated that mRFP1-expressing rhesus monkey cells were diploid and had a normal chromosome complement (42 chromosomes; Figure 1c). This approach offers the advantage of simple visual confirmation of rhesus monkey gene expression in iSCNT embryos, without compromising embryo viability (Figure 2a, b). We treated embryos with three concentrations of RepSox (5, 25, and 50 μM) to determine its potential influence on rhesus monkey-pig iSCNT embryo development. There was no significant difference in the cleavage rate (73.6, 72.2, and 70.4%) compared with the untreated group (72.4%, Table 1). The efficiency of blastocyst development was slightly higher in the 25 μM RepSox-treated embryos than in untreated embryos, but this was not significant (Table 1). When iSCNT embryos were treated with RepSox for different durations, development to the blastocyst stage was higher in the group treated with 25 μM RepSox for 1 day (24 hours) than in the control group (1.2 vs. 2.4%; Table 2). There was no significant difference in the total cell number/blastocyst among the groups (Table 2; Figure 2c). Embryos were treated with 25 μM RepSox for 24 hours in the following experiments.
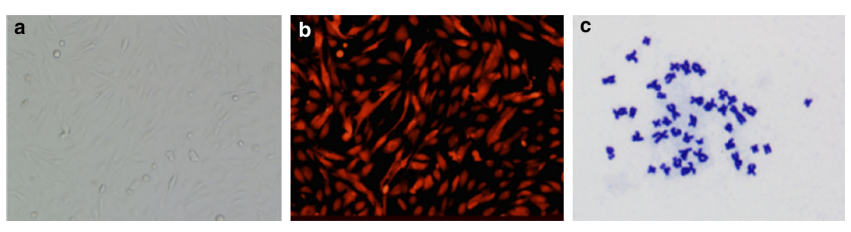
Figure 1. mRFP1-expressing rhesus monkey cells used as donor cells. (a) Bright-field image of mRFP1-expressing rhesus monkey cells. Original magnification, ×100. (b) Red fluorescence in mRFP1-expressing rhesus monkey cells. Original magnification, ×100. (c) Karyotype analysis of a rhesus monkey cell showing a diploid number of chromosomes. Original magnification approximately ×1000.
Figure 2. RepSox-treated mRFP1-expressing rhesus-porcine monkey iSCNT embryos at the blastocyst stage. (a) Visible light image of blastocysts derived from RepSox-treated rhesus monkey-pig iSCNT embryos. (b) Blastocysts derived from RepSox-treated rhesus monkey-pig iSCNT embryos showing red fluorescence under UV light. (c) Hoechst 33342 staining of a RepSox-treated rhesus monkey-pig iSCNT blastocyst. Bars represent 100 μm.
The Influence of Culture Medium on Rhesus Monkey-Pig iSCNT Embryo Development
The developmental capacities of RepSox-treated rhesus monkey-pig iSCNT embryos cultured in three types of culture media (NCSU-37, HECM-9, and iPSC medium) were compared. The cleavage and blastocyst formation rates did not significantly differ among the three groups (Table 3). The culture media did not influence the development of rhesus monkey-pig iSCNT embryos.
Detection of Oct4 in RepSox-treated iSCNT Embryos at the Blastocyst Stage
Figure 3 shows immunostaining for Oct4 in rhesus monkey-pig iSCNT embryos at the blastocyst stage. The fluorescence intensity of Oct4 staining was significantly higher in RepSox-treated embryos than in untreated embryos (p < 0.05, Figure 3).
Figure 3. Oct4 levels in rhesus-porcine monkey iSCNT blastocysts with or without RepSox treatment. (a) Images of immunostaining for Oct4 (green) in rhesus monkey-pig iSCNT blastocysts without or with RepSox treatment. Each sample was counterstained with Hoechst (blue) to visualize DNA. (b) Quantification of Oct4 signal intensities in rhesus monkey-pig iSCNT blastocysts without or with RepSox treatment. M-P RepSox (-): iSCNT blastocysts not treated with RepSox, M-P RepSox (+): iSCNT blastocysts treated with 25 μM RepSox for 24 hours. Significant differences between groups are indicated as *p < 0.05.
Effects of RepSox on mRNA Expression in iSCNT Embryos
Due to the poor blastocyst formation rate, the relative mRNA expression of pluripotency-related (Oct4, Sox2, and Nanog) and apoptosis-related (Bax and Bcl2) genes was evaluated at the 2-4-cell stage (Figure 4). Relative expression of Oct4 and Nanog was significantly higher in RepSox-treated embryos than in untreated embryos (p < 0.05). However, relative expression of Sox2 was similar in RepSox-treated and untreated embryos. By contrast, expression of the anti-apoptotic gene Bcl2 and the pro-apoptotic gene Bax was higher in RepSox-treated embryos than in untreated embryos. However, there was no difference in the relative Bax/Bcl2 ratio between the two groups.
Figure 4. Relative mRNA levels of pluripotency-related (Oct4, Nanog, and Sox2) and apoptosis-related (Bax and Bcl2) genes in rhesus monkey-pig iSCNT embryos at the 2-4-cell stage with or without RepSox treatment. Relative mRNA abundances of various important genes in rhesus monkey-pig iSCNT embryos at the 2-4-cell stage. M-P RepSox (-): iSCNT embryos not treated with RepSox, M-P RepSox (+): iSCNT embryos treated with 25 μM RepSox for 24 hours. Values with different superscripts within groups are significantly different (p < 0.05). Values are the mean (± standard deviation of the mean) of four independent experiments.
DISCUSSION
Although embryos of a number of species have been successfully cloned, incomplete nuclear reprogramming remains the major obstacle for iSCNT (Rideout et al. 2001; Shi et al. 2003; Zhang et al. 2014). In the present study, we investigated the effect of RepSox on nuclear reprogramming of rhesus monkey-pig iSCNT embryos for the first time, with the aim of regulating expression of important development-related genes and improving the reprogramming capacity of iSCNT embryos.
Successful embryonic development is dependent on the proper expression of specific genes, including Oct4, Nanog, and Sox2, which are closely associated with pluripotency and early embryonic development (Zhang et al. 2014). Expression of Oct4, Nanog, and Sox2 is lower in SCNT embryos than in IVF embryos (Latham 2005). Therefore, improving the expression of pluripotency genes in iSCNT embryos is one strategy to modulate nuclear reprogramming. In the present study, RepSox treatment significantly improved the mRNA expression of Oct4 and Nanog in iSCNT embryos at the 2-4-cell stage (Figure 4). Consistently, treatment with another small molecule, sodium butyrate, significantly increases the expression level of Oct4, although the expression of other pluripotency-related genes was not assessed in that study (Xiong et al. 2015).
We believe that these changes in expression of Oct4 and Nanog would improve epigenetic reprogramming of iSCNT cloned embryos. However, inconsistent with the changes in Oct4 and Nanog expression, Sox2 expression did not differ between RepSox-treated and untreated embryos (Figure 4). This might be because RepSox can replace, but not enhance the expression of Sox2 during iPSC reprogramming (Ichida et al. 2009). Due to the poor blastocyst formation rate, we were unable to assess gene expression at the blastocyst stage. Oct4 is a very important gene, the low level of somatic cell reprogramming during preimplantation development of murine-porcine iSCNT embryos is due to a lack of Oct4 protein expression (Jiang et al. 2011). Immunostaining for Oct4 at the blastocyst stage showed a higher intensity in RepSox-treated embryos than in untreated embryos (Figure 3). RepSox might be able to regulate Oct4 expression throughout preimplantation of rhesus monkey-pig iSCNT embryos.
RepSox partially rescued the expression of specific genes, which would be expected to improve iSCNT embryonic development. The blastocyst development rate was higher in the group treated with 25 μM RepSox group for 24 hours than in the untreated group. However, this was not significant (Tables 1, 2). Other studies showed the same outcome. Trichostatin A does not have any beneficial effect on the development of gaur-bovine (Jiang et al. 2011), human-rabbit (Shi et al. 2008), and dog-porcine (Sugimura et al. 2009) cloned embryos. We concluded that the slight improvement of development in RepSox-treated rhesus monkey-pig iSCNT embryos may be related to the significantly increased levels of these important development-related genes during in vitro development.
CONCLUSIONS
Treatment with 25 μM RepSox for 24 hours improved the expression of important pluripotency-related genes (Oct4 and Nanog) at the early developmental stage in rhesus monkey-pig iSCNT embryos in vitro. RepSox treatment also greatly improved the immunostaining intensity of Oct4 at the blastocyst stage; however, rhesus monkey-pig iSCNT blastocyst development was not significantly improved. It is reasonable to speculate that cloned embryos need to undergo extensive changes in terms of various epigenetic modifications and their gene expression profiles to restore complete nuclear reprogramming. Thus, RepSox is a suitable chemical candidate to induce nuclear reprogramming by regulating the expression of important development-related genes in iSCNT embryos. Combined treatment with RepSox and regulators of other epigenetic modifications may facilitate complete epigenetic reprogramming and promote the development of iSCNT embryos.